What is the difference between inclusion and exclusion criteria? How do you know whether something should be an inclusion or exclusion criterion for your study?
In a prospective study, you define the study population and enrol eligible subjects before applying the intervention – a medication, treatment, surgical procedure, or placebo intervention.
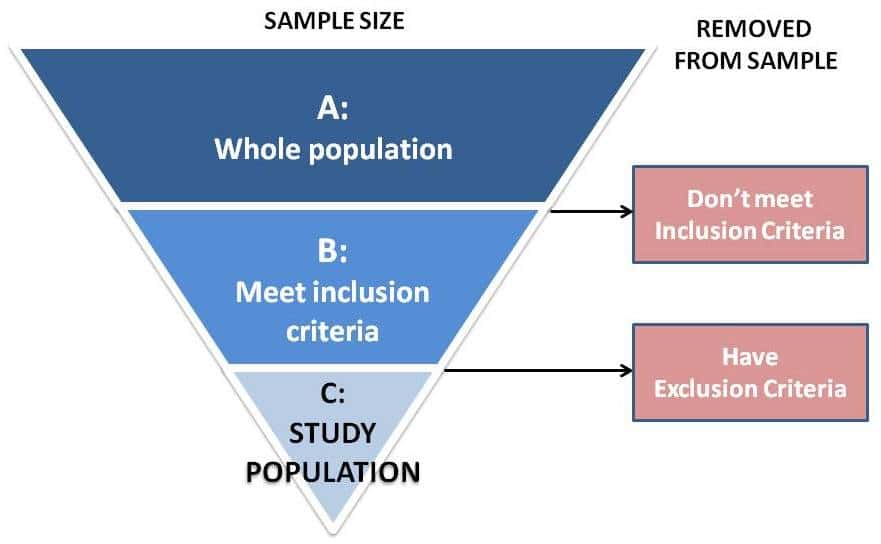
First define where the population will be drawn from, such as all new patients seen by a doctor, or all people admitted to a hospital emergency room, during a given period of time, such as between January 1, 2019 and September 30, 2019. This is your whole population (Bar A in Figure below).
Looking at this population as a whole, define criteria that subjects must meet to be eligible for the study – these are the inclusion criteria. Examples:
Adult age.
Able to give informed consent.
Have a certain condition, such as cancer or arthritis.
Be at a particular stage of the condition, such as stage 4 lung cancer.
All nonoperative treatments must have failed.
After you’ve applied the inclusion criteria and removed any subjects who don’t meet these criteria, you’re left with a smaller population set of all potentially eligible subjects who have met all of the inclusion criteria (Bar B in Figure below).
Then you apply the exclusion criteria – other subject characteristics that you know or strongly suspect are likely to affect the outcome, sometimes called confounding variables. Examples:
Comorbidities such as diabetes, hypertension, or neuroarthropathy.
Use of certain medications.
Previous surgery at the surgical site.
Additional or multiple treatments or surgeries required.
Pregnancy.
Smoking or alcoholism.
Inability to complete the measured outcomes.
After you’ve applied the exclusion criteria, you are left with the actual study population that will receive the treatment, control treatment, or placebo (Bar C in Figure below).
Sometimes inclusion and exclusion criteria seem like two sides of the same coin. Use positive language to describe both. Inclusion criteria are the broader strokes, the must haves. If the subject must not have something, that’s an exclusion criterion. For example, one could say an inclusion criterion is that the subject “must not be pregnant” (negative language). It is easier to say an exclusion criterion is pregnancy (positive language).
During the course of a study, some of the eligible patients may decline to participate in the study, may decline treatment, may have incomplete data because they answered one questionnaire but not another one, may die for causes related or unrelated to the study, may move away, or may simply drop out of the study. These patients are considered “lost to follow-up” in a prospective study.
A retrospective study evaluates treatment outcomes or the effect of certain parameters on outcomes for a treatment that was performed on a given population at some point in the past. Retrospective studies typically include chart reviews of patients previously treated by a given doctor or hospital, or mining the data from a large regional or national patient registry database.
In a retrospective study, the whole population is defined by the database within a given time frame (Bar A in Figure below). Then inclusion criteria (the must haves) and exclusion criteria (the must not haves) are applied the same way as in prospective studies.
Some items that would be considered “lost to follow-up” in a prospective study can be listed as additional exclusion criteria in a retrospective study. For example, a follow-up period of less than X months or years, or missing data, can be listed as exclusion criteria for a retrospective study, because you’ll review these parameters at the outset and exclude these subjects from the study before you actually collect and analyze the data.
Hope this helps! Feel free to leave a question or comment. I also welcome suggestions for future weekly writing tips.


Recent Comments