The purpose statement of a manuscript is the most important sentence in the paper. The entire manuscript is constructed around and flows from the purpose statement. If the purpose of your study is not clear, the reader will have difficulty understanding the need for your study and appreciating the meaning, relevance, and importance of your study.
Ideally, the purpose statement should be a separate paragraph – the final paragraph of the Introduction. Sometimes the purpose statement is the final sentence of the closing paragraph of the Introduction, where the rest of the paragraph sets up the intent for the study in more detail.
Constructing an effective and complete purpose statement takes a bit of practice – but once you’ve got the hang of it, this becomes an easy process, perhaps even formulaic.
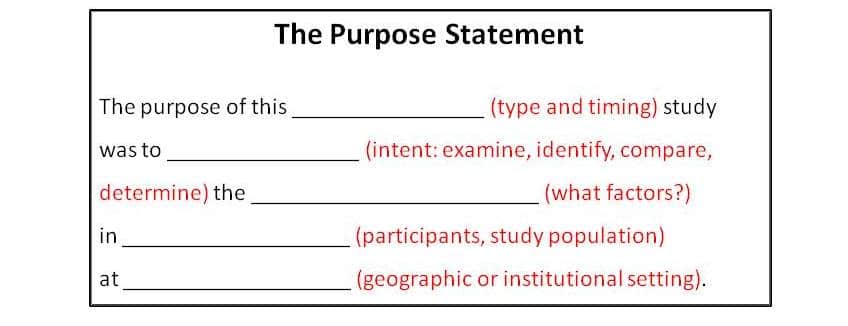
A good purpose statement not only tells the reader WHY you conducted the study, but also WHAT you studied, and HOW you went about studying it. The best purpose statement does not get creative; rather, it lays out these essential points in a straightforward, simple, logical manner.
The purpose statement is usually written in passive voice, as the focus should be on the study, not who did it. And it is written in past tense, since you conducted the study in the past, it is completed now, and you’re reporting on what you DID, not what you’re doing now.
1. Opening Lead-In
I always start the purpose statement with “The purpose/aim of this study was …” It may seem mundane, but it’s direct, succinct, and makes it easy for the reader to find the purpose statement.
If the purpose statement is for an abstract (either for the manuscript, or for a conference) and word length is a concern, it’s acceptable to switch to active voice and begin the sentence with “We studied/ explored/ determined/ compared/ …”
2. Qualify the Type of Study (How?) and the Timing (When?)
The reader needs to know what type of study this was. Was it quantitative, qualitative, or mixed methods? If it’s a quantitative study submitted to a journal that publishes mainly quantitative studies, or a qualitative study submitted to a journal that publishes mainly qualitative studies, then you don’t need to specify this. Rather, provide a more specific descriptor, such as randomized clinical trial, matched cohort study, case study, systematic review and meta-analysis, ethnographic, grounded theory, focus group, etc.
If the methodology is complex, such as for a propensity score matched-cohort analysis or mixed methods, it is probably best to introduce the methodology to the reader in a preceding paragraph in the Introduction and give examples of how other studies in your general research area have used this methodology.
The reader also needs to know whether the data were collected prospectively, or had been collected previously and were analyzed retrospectively.
Examples:
The purpose of this randomized, controlled clinical trial was …
The purpose of this randomized, controlled, clinical, feasibility, pilot study was …
The purpose of this retrospective chart review was …
The purpose of this prospective cohort study was …
The purpose of this retrospective case series was …
The purpose of this scoping review was …
The purpose of this longitudinal, retrospective cohort study was …
Note that a randomized clinical trial is, by its very nature, always prospective, so to state it is a prospective, randomized trial is redundant (see a previous blog post on common redundancies).
3. The Intent of the Study (What?)
What was the intent of this study? What did you want to accomplish? Did you analyze, determine, evaluate, describe, or compare? Use the appropriate verb, and describe the objective or item you wanted to achieve.
Examples:
… to examine demographic characteristics, management and outcomes in …
… to identify research gaps in the existing literature regarding …
… to assess the safety and effectiveness of …
… to determine intermediate to long-term outcomes of …
… to compare preoperative function and quality of life in …
… to determine the most relevant gait parameters and clinical outcome measures …
… to determine if the cost of Procedure A was similar to the costs for Procedures B, C, and D
4. The Participants and Conditions (Who?)
The participants in the study need to be mentioned. Describe the sample cohort and/or the population, with the main defining criteria for inclusion. Detailed descriptions of inclusion and exclusion criteria will be provided in the Methods, but the reader should understand who the sample population or study participants were, in general terms, from the purpose statement.
Examples:
… in individuals at moderate risk for future fracture …
… in patients before and after total hip arthroplasty …
… in people with knee osteoarthritis …
… in people with uncontrolled diabetes …
… in individuals on statin medications for greater than two years duration…
… in individuals who had a myocardial infarction within the last six months …
5. Additional Information
Depending on the complexity of the study, if the purpose statement is not too cumbersome at this point, it’s helpful to include additional information such as the setting of your study, i.e. the type of institution (large urban hospital) or department (emergency room, fracture clinic) and/or geographic location, if this is relevant to your study or distinguishes it from published studies.
6. Primary and Secondary Outcomes
In step 3, you stated the general intent and a general description of the outcomes that were examined. However, the purpose statement should identify the primary and secondary outcomes. It’s particularly important these are defined for randomized clinical trials, other prospective studies, and studies with extensive statistical analyses. These can be described in a second sentence:
“The primary outcome was the rate of joint fusion; secondary outcomes included patient-reported outcome measures and walking speed.”
Ok, once you put it all together, you have complete purpose statements:
The purpose of this prospective cohort study was to determine intermediate to long-term outcomes of total hip arthroplasty using the X prosthesis at two Canadian centres.
The purpose of this randomized, controlled, feasibility, clinical pilot study was to determine the most relevant gait parameters and clinical outcome measures when assessing the effectiveness of polyol-containing hyaluronic acid injection in people with knee osteoarthritis.
The purpose of this study was to prospectively assess the safety and efficacy outcomes for the synthetic cartilage implant population and to determine if the outcomes achieved at two years were maintained at more than five years.
The purpose of this retrospective chart audit was to investigate the prevalence of vitamin D deficiency and other secondary causes of osteoporosis in a selected cohort of patients with a fragility fracture who were referred to a metabolic bone disease clinic for further evaluation.
The purpose of this scoping review was to examine demographic characteristics, management and outcomes for individuals at moderate risk for future fracture.
I hope you found this Weekly Writing Tip helpful and feel less intimidated about constructing a comprehensive and powerful purpose statement. I’d love to hear how you put these tips into practice. I also welcome suggestions for future #WeeklyWritingTips.


Recent Comments